ITECH's EU Bet
Will they let Selektope sail?
Introduction
I been a shareholder of ITECH for quite a while and I am very impressed at the amount of misconceptions around this company and I think it is time to put our research glasses on and tackle a crucial one…..
My goal here is to help existing shareholders understand this catalyst better and for interested investors to be able to assess the situation better and make an informed decision based on data and not rumours/emotions.
Information about this catalyst was fragmented in multiple sources!
Let’s get straight into it, I am super excited to share my research findings here!!!
Some proper background
Before we proceed in the examination of this catalyst we need to get a fundamental understanding of what the business we are examining,
ITECH is located in the very beautiful country of Sweden, and they the first company to apply principles from bio technology research in the paint industry to keep ship hulls free from marine fouling.
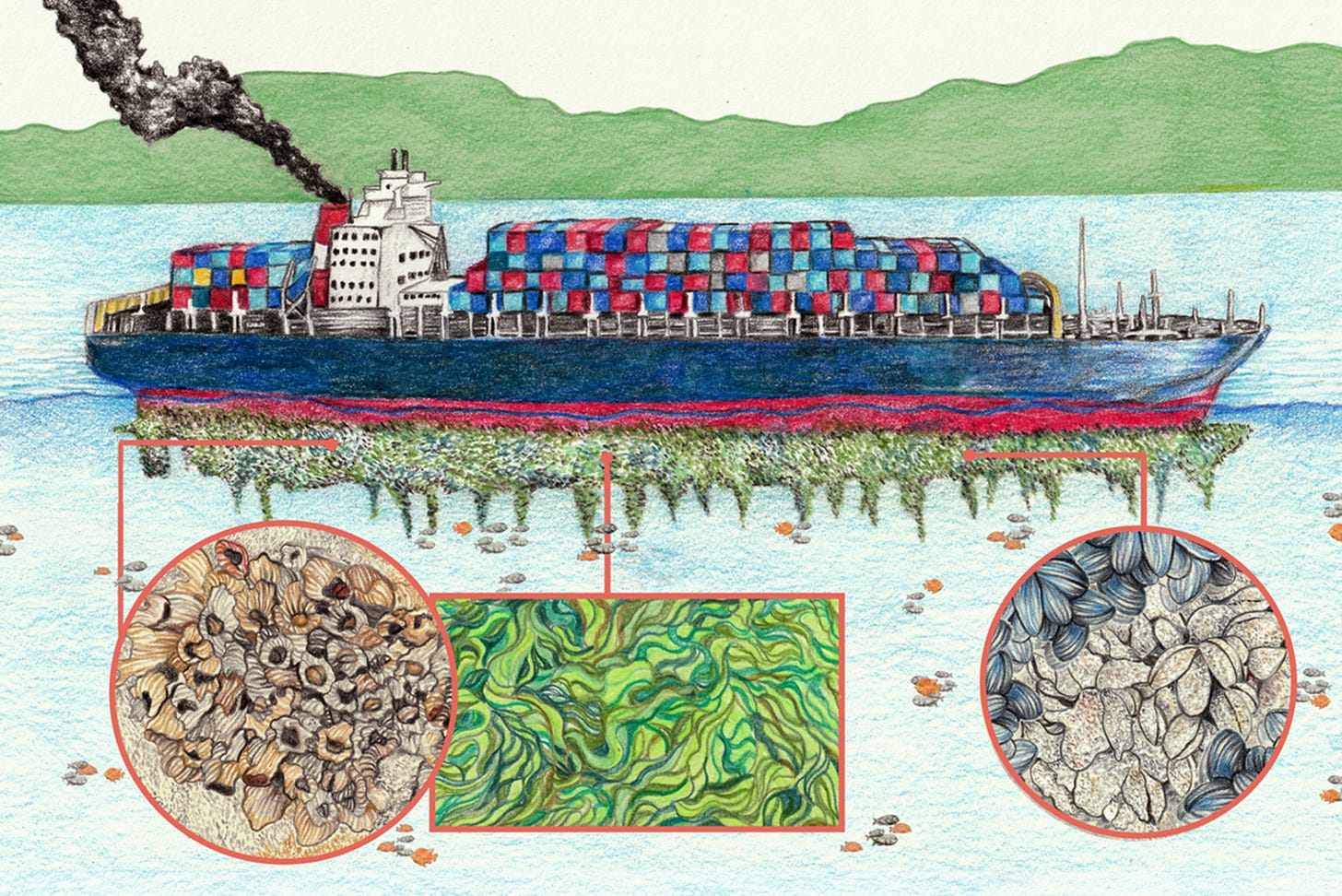
What is marine fouling I hear you ask, glad you asked!
Biofouling is the process that begins with submerging any clean surface in water, then organic and inorganic substances start forming in the surface, then bacteria and algae colonise the surface to form the primary biofilm, this is called the slime layer. This becomes the foundation for something much worse.
Hard fouling —> In prolonged submerged times (always the case for ships) this situation transforms in hard fouling which is formed by the attachment of barnacles in the submerged areas of the ship, this phenomenon mainly appears in the hull area.
Once one stick then usually the rest grow like rapid fire as a single barnacle parent can release up to 10-20 THOUSAND larvae that survive for weeks.
Illustration: Ricardo Macía / China Dialogue Ocea
How big of a problem? Massive actually, let me explain
Even moderate hard fouling about 10% barnacle coverage, can force a ship to use 36% more shaft power to hold speed. In severe calcareous fouling, the power penalty can climb to 86% at cruising speeds.
Besides being a big eyesore, barnacles can compromise the boats integrity, as well as drastically slow them down due to strong drag resistance.
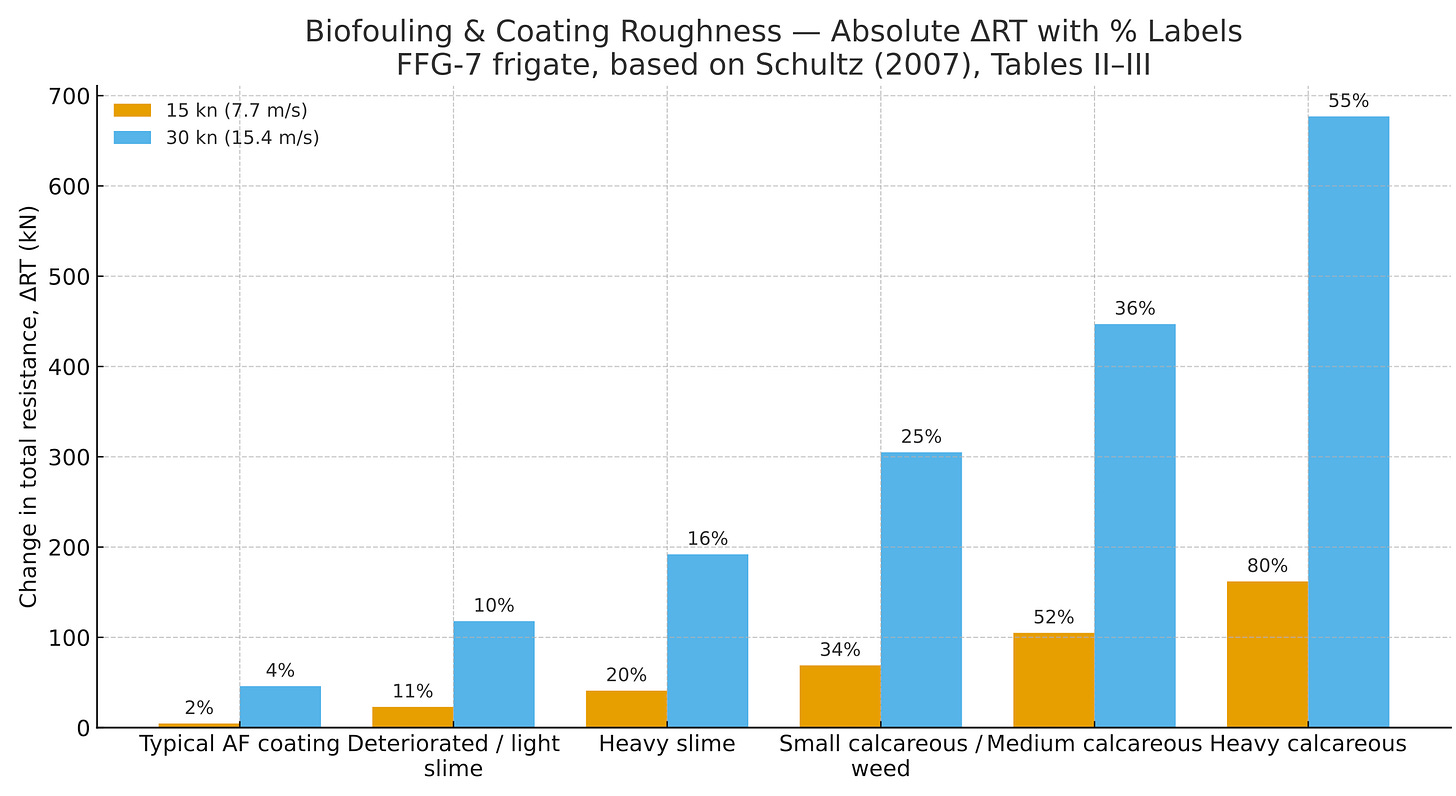
This board showcases the massive effects of the dragging in 2 different speeds, 15 knot & 30 knot which is the speed of the vessels
X axis = Stages of biofouling, Y axis = Resistance measures by both studies in kilo-newtons
The percentages on top of each bar are how much resistance % increases at each stage of the biofouling, the effect is much larger at 30 knots in absolute terms, but it’s a smaller percentage because the total (smooth) resistance at high speed is huge, wave-making/residuary resistance grows rapidly with speed, so friction/roughness makes up a smaller share of the total.
Bigger resistance = More energy needs = Increased fuel usage = Increased costs for ship owners
To put this into perspective ITECH studied 685 ships that came for dry-docking, 33% of them had above 10% of their area submerged in water was covered with barnacles (which is considered unacceptable levels), this resulted in over 36% need for higher power required to maintain the same speed as compared to a clean hull!!
But there is an even bigger issue with increase fuel consumption, environmental goals are much harder to meet and they are required by the EU. The punishments are harsh from risking fines even to begin denied access in EU ports. Shipowners are incentivised to keep emissions and environmental pollution as low as possible
But how can they do that?
Dual value proposition - Speed Improvement/Efficiency
Their solution?
The Selektope chemical!
It is a chemical with unique properties, it utilises Medetomidine (super important for later). When it is integrated in the ship paint (only 2 grams needed per litre, very effective) barnacle larvae are temporarily affected only when they approach the painted surface. The effect is that larvae become hyperactive and cannot attach to the surface of the hull, instead they are forced to swim away and find another place to settle, this happens through natural receptor stimulation from medetomidine, activating their swimming mode, without hurting the larvae.
Here is a video from Itech’s that shows in a simple way how Selektope works
Dark Cloud’s appear
In 2023 (yes our story has deep lore!), BPC (Biocidal Products Committee) concluded that medetomidine should be classified as an endocrine disruptor (ED) for humans, and did not support a straight renewal of Selektope under the Biocidal Products Regulation (Source - ECHA).
The main issue being ‘‘ “traditional exposure assessment revealed no significant risks, except for young children who may come into contact with freshly treated surfaces.” (ECHA)
To Quote them directly
The BPC has concluded that medetomidine is not mutagenic, carcinogenic, or a reproductive toxicant. However, it does exhibit endocrine-disrupting properties that affect and related biological pathways. Although there are no standardized methods for assessing these effects, medetomidine is categorized as an endocrine disruptor under EU regulations. Traditional exposure assessment revealed no significant risks, except for young children who may come into contact with freshly treated surfaces. However, a definitive conclusion regarding the overall risk to human health remains elusive.
In chemistry (yes I went that deep) medetomidine is a potent adrenoceptor agonist – the actual property that makes it a sedative in mammals is by reducing release of norepinephrine (a stress hormone).
The argument being that this mechanism, at certain exposures, constitutes an endocrine mode of action capable of disrupting normal hormonal signaling in an intact organism (in this case humans). Indeed, studies in humans and animals have shown that medetomidine exposure and similar α<sub>2</sub>-agonists can alter levels of cortisol, insulin, and other hormones as a downstream effect of sedation (European Journal of Endocrinology Issue 1).
Initial Reaction & How I-TECH is fighting back
The initial reaction when this was first published was a 50% drop in share price, since then a lot have changed,
The reaction was very quick from the company and it rejected the ED characterisation in the two key fronts
Law —> Under the EU’s biocides regime, a substance is only an endocrine disruptor for humans if all three legal criteria are met: (i) an adverse effect is shown in an intact organism, (ii) an endocrine mode of action (MoA) is demonstrated, and (iii) the adverse effect is a consequence of that endocrine MoA.
Science —> Medetomidine’s well-known pharmacology is α₂-adrenoceptor agonism, the sedative response at pharmacological doses is completely reversible once exposure stops and leaves no permanent effect. I-Tech’s position is that the observed sedative and post-sedative physiological changes are secondary, non-endocrine consequences of transient pharmacology (and not direct effects on an endocrine organ or a lasting alteration of endocrine system function). In other words, while adrenergic signalling can modulate hormones, the evidence in the file does not show a harmful, persistent endocrine disruption that satisfies the legal “adverse-effect-via-endocrine-MoA” chain required to label the substance an endocrine disruptor.
The Eu commission in September & November of 2024, created a public consultation to understand if the criteria for derogation are met for Selektope, ITECH successfully rallied close to 50 stakeholders (ITECH annual report 2024) and they submitted information in the highlighting the importance of antifouling biocides to reach the CO2 emission targets for EU, the limited availability (good argument) of biocidal and non-biocidal alternatives effective against hard fouling and how a non-approval would have a significant impact on competitiveness for shipowners based in EU. Stakeholders engaging covered both EU and global trade organisations, individual paint manufacturers, ship owners, active substance suppliers and antifouling industry.
Not only that but the company hired third party independent consultancy to make a report about the socioeconomic effects of banning the substance, plus more assessments were made to be sent in the EU to have a complete and accurate view of the situation. I have gathered a list of all possible outcomes and strong arguments in each area of the legal aspect. More on that later!!
Recent developments from the Q2 2025
I-Tech AB announced that the current approval for Selektope (Medetomidine) in the European Union has been extended by one year to the 30th of June 2026 (EU Journal) This extension is part of the normal procedures as the EU Commission continues its thorough reregistration process. While the EU Commission's decision is taking longer than initially anticipated, I-Tech views this extension positively.
Markus the CEO, commented that ITECH team also met with the Commissioner’s Cabinet to provide specific feedback on factual errors in the ongoing Selektope re-registration case. Next step of the process, which is the next quarterly meeting of the Standing Committee on Biocidal Products (SCPB) in September. (Q2 2025, Call transcript)
This marks the second extension given by the EU, with the first one being for 2.5 years, EU Journal
So this catalyst is still in development and will more than likely be resolved within 2026-2027.
Clarity into the situation
I usually prefer to always, put the data into the table and decide the significance of every catalyst with a clear mind, this is by far the most effective way to approach complex catalysts like these,
Their strengths
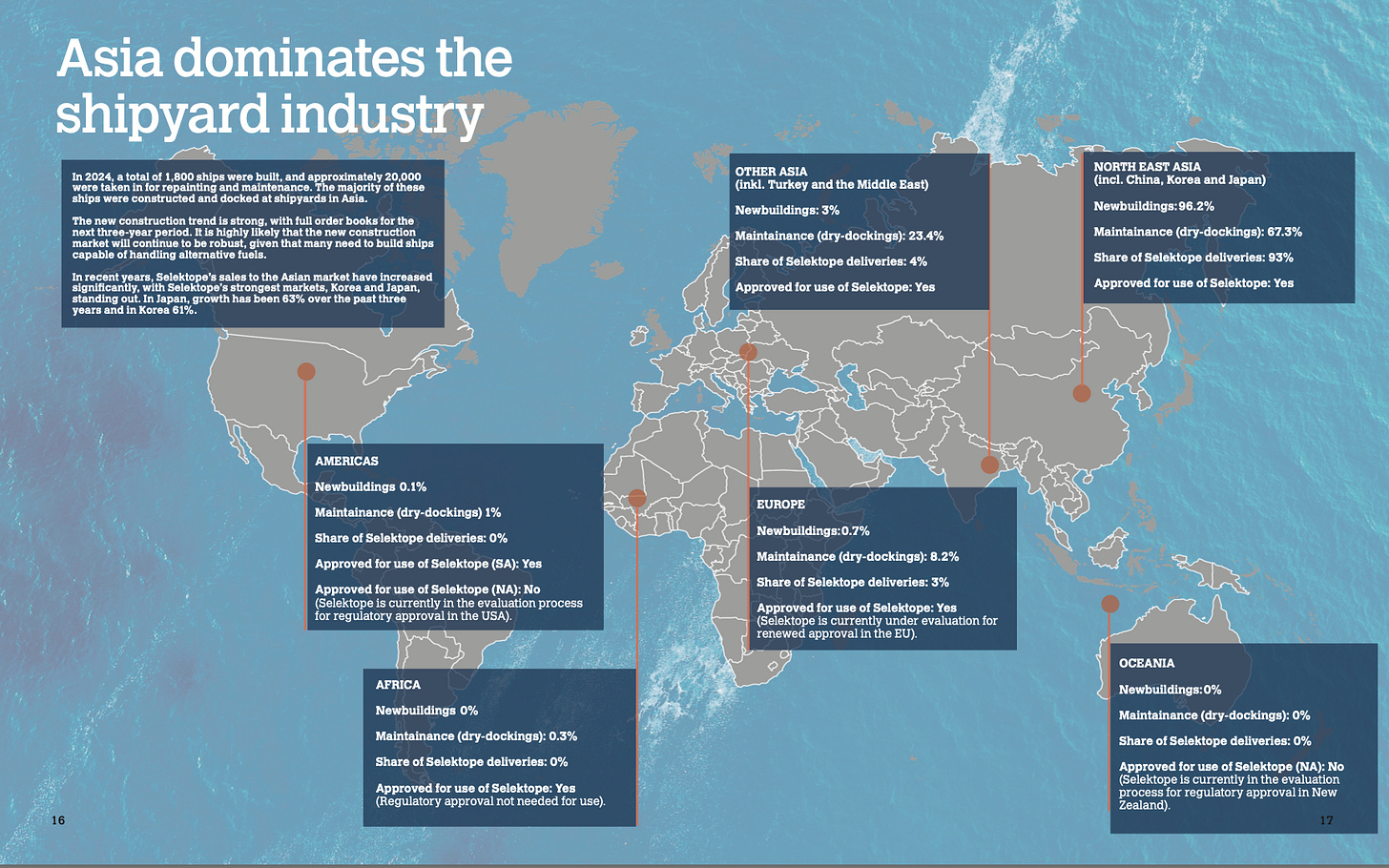
As things stand now, even in the worse case scenario Europe represents a minority of their revenue, so the growth aspect of the business is still intact
Out of their sales 90%+ (depending on the quarter) are in Asia and not in Europe , they have strategically positioned themselves in these markets which are going to be the biggest drivers of growth.
From their 2024 Annual Report
In Q2 two important things were noted but not given enough attention due to the results, they are strengthening their presence in Korea & they are hinting a new product reached major technical milestones to further diversify revenues (unfortunately not more details were given)
EU has delayed the decision over and over again, I view this hesitation positive as they are usually quick and decisive with their legislation process
Current view of the medetomidine being an ED is not favoured in the rest of the territories in which they operate, plus regulation isn’t that strict
The minus
There have been occasion in the past were the EU commission banned the substance, TBT was the most prolific case of an antifouling ban, so it has happened in the past if certain criteria aren’t met.
If the ban happens it’s not a fatal scenario but it would make it hard to expand as the technology could be stigmatized & would be harder to convince new clients
Regulatory hurdles for ED are very strict within the EU as I observed in older cases
EU is very obsessed with regulation versus innovation
But now let’s examine a bit more in depth shall we?
The main counters are on these 3 fronts
a)There is negligible risk to humans, animals of the environment
b) There is negligible risk to humans, animals of the environment; the active substance is essential to control a serious danger to human health, animal health or the environment;
c) not approving the active substance would have a disproportionate negative impact on society.
So in my opinion the strongest arguments are the following,
Guidance on the identification of ED substances under the BPR[2] states that, in the absence of internationally validated test methodologies, no specific guidance can be given here on how to identify potential links between such [endocrine] effects to non-EATS endocrine modalities.
It is well known and extensively documented that Medetomidine, at pharmacological concentrations, activates the alpha2-adrenoreceptors that we discussed earlier. This is the desired pharmacological response for which Medetomidine has been used for the last 20 years and sedative effects are reversible upon removal of medetomidine exposure. (Press release, May 24)
ITECH argues that their post-sedative effects are considered not to be a direct action of medetomidine. Most notably, there is no clear direct effect of Medetomidine on an endocrine organ or tissue and whilst the function of the endocrine response may be moderated, the actual function is not fundamentally altered and cannot be considered adverse (ITECH’s white paper on Medomidine).
In fact, according to a study by Calypso (a ship operator), effective antifouling systems, such as those enabled by Selektope, save the shipping industry over 100 million tonnes of carbon dioxide annually.
Ship owners, paint manufacturers, and even climate advocates have largely rallied in support of Selektope’s continued use and for good reason. During ECHA’s public consultations, stakeholders emphasised that Selektope (medetomidine) is irreplaceable in its role.
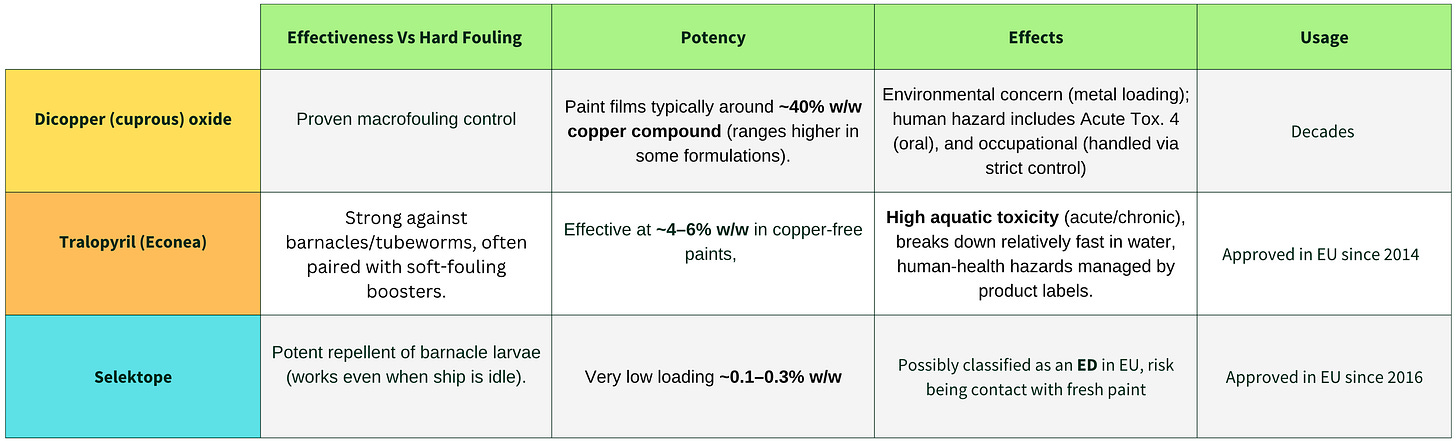
It is not well known that only two other biocidal compounds globally exist copper oxides and tralopyril that provide comparable antifouling protection against barnacles and hard fouling, and those come with their own environmental drawbacks as we are going to discuss in a bit. Everyone stressed a “lack of suitable alternatives” if medetomidine was banned by the EU. Selektope aligns with the EU’s green shipping and climate goals by cutting fuel usage and emissions.
Specifically, whilst the AoA concluded that there were many suitable alternatives to medetomidine in PT21, stakeholders observe that, in reality, only three active substances have principal activity against ‘hard fouling’. The remaining substances are acknowledged to tackle mostly soft fouling’(as we explained in the opening of the article) and would always need to be formulated alongside an active substance with principal activity against hard fouling in an anti-fouling paint.
Currently only three substances with principal activity against hard fouling (dicopper oxide, copper thiocyanate and tralopyril), copper thiocyanate is noted to be used primarily for leisure craft rather than large commercial vessels which are the main clients of ITECH.
This means in actual practice, only two alternatives to medetomidine are dicopper oxide and tralopyril that have a comparable functionality and use profile. It is noteworthy that none of the stakeholder comments available to I-Tech supported the conclusions of the assessment of alternatives reported in the BPC opinion.
Here is a comparison board to compare the different solutions available to tackle hard fouling
Dicopper is used mostly as an all around solution while ITECH is prominent in barnacle prevention in idle situations,
The idle aspect, which ITECH shines is more crucial than it seams, idle time in warm waters massively raises barnacle risk. IMO’s 2023 Biofouling Guidelines flag extended idle time as a key driver, with sensitivity depending on coating type. Tankers & bulkers queuing/at anchorage, offshore support & shuttle tankers, project/heavy-lift rely on Selektope to stay barnacle free and decrease fuel consumptions. Non-biocidal hull coatings are not technically feasible on all commercial vessels. There is a lack of suitable alternatives here!
According to the Q2 call transcript in Sweden, there has been some, very noteworthy, spotting of new marine species. And this is, of course, threatening the biodiversity of our European coastal waters.
The number one source for spread of invasive species is actually the fouling of ship’s hulls. Especially in the big areas of the hull because that is implications for the fuel consumption. But actually, ships have also problems in these so called niche areas, which are very difficult on one hand to service and maintain, but also very, very difficult to clean during the dry docking cycle. Then you have the possibility for invasive species to thrive and damage ecosystems. (Markus Q2, 2025)
Interestingly..
In the banning case of TBT (anti-fouling substance) the effect of the substance usage of tributyltin, the ED properties were extreme & actually visible, notably triggering imposex in marine snails (females developed male sexual organs), which devastated shellfish populations. It also accumulated up the food chain, posing risks to marine mammals and even humans. Researching for this article I wanted to see how the committee handled similar cases, and here no multiple 1 year extensions were given, rather a set date. Governments agreed in 2001 to stop applying organotin biocides by 1 Jan 2003 and to ensure all ships had removed or sealed them by 1 Jan 2008.
Judging also from the behaviour of the committee in most cases when there are multiple 1 year extensions that usually does not suggest a ban, in 14 cases in recent history, that the committee examined and gave more than 2 times the one year extensions 79% of them resulted in renewal or renewal with restrictions, this of course does not guarantee a result but it is an interesting statistic, that said everything can happen in those complex legal procedures.
Also ECHA’s even though claimed ED properties, in their process it has mostly highlighted that the risk comes from direct contact with freshly painted surfaces (e.g., small children touching hulls soon after application) but not exposure via seawater . Occupational exposure for painters is also a risk that could be managed (PPE, curing times). The Commission’s file confirms medetomidine has an ED (human-health) hazard, but the exposure scenario of concern in practice is contact with fresh paint the routine public exposure via the water was not identified as a significant risk in traditional exposure assessments (EU Journal).
So the practical concern is direct human touch of the paint not in water use, which is something I saw many people confuse.
Possible outcomes of the situation
A. Full Renewal Approval
This is the outcome ITECH is fighting for, in this instance, the EU Commission would renew medetomidine in PT21 by overriding the ED “exclusion” via Article 5(2) BPR (derogation), despite the file recording that medetomidine meets the endocrine-disruptor criterion.
This would take a lot of effort as a very strong showing is required on negligible exposure (pro-only application, curing/label controls) and/or a compelling societal impact case (disproportionate negative consequences of non-approval as we discussed above) under Art. 5(2). Worth noting on older cases in chemicals with ED properties this is rarely the case.
B. EU Ban
A non-renewal Decision citing the Article 5(1) BPR (ED = exclusion, so “shall not be approved”), similar to the case with acrolein PT12 (2023) and sulfuryl fluoride decisions after long evaluations.
This would be the worst case scenario but as shown above not fatal to the company contrary to what most believe (very interesting topic for a potential deep dive). If this plays out it will be likely in the form of stop all substance usage by x date like it happened with the banning of TBT. Given the benefits of Selektope & data I believe the % of this taking place are low.
C. Time Limited Approval
A shorter renewal (often <5) with candidate-for-substitution framing and an explicit review clause. This model has been observed in previous cases and it is how the EU handled some chemical renewals before (e.g., coumatetralyl) . Creosote PT8’s 2022 renewal also demonstrates tightly circumscribed, time-bound approvals from the committee.
D. Strict Approval with limitations
A renewal under Article 5(2) with tight conditions e.g., professional-only use, application/curing controls, labelling to prevent child contact, possibly monitoring and a shorter term. This mirrors how the Commission has renewed other similar cases like Creosote.
In their own summary notes no significant risks exist in traditional exposure scenarios except for potential contact of young children with freshly treated surfaces, in my personal view this type of risk you can control with pro-only + curing/labels.
We could also see a combination of C + D, stricter rules in usage and reviewed for renewal more frequent.
My final thoughts/opinion - There are multiple strong arguments to be made for the renewal of the approval of Selektope as it has unique benefits for it’s clients, tough to argue that there is a proper substitute out there. While we cannot be certain of the outcome of the EU decision I like how they have given emphasis on geographical diversification, how they have acted rallied shareholders and provided strong arguments to the commission for their case. Their Asian presence alleviates the potential negative impact of the catalyst.
In my personal opinion the arguments make a compelling case for the renewal. I am still monitoring the situation closely and this article will get constantly updated in case new data arrives.
For any questions about this article or the company feel free to comment or reach out via a direct message!
Write your thoughts down, I will be responding to everyone!
If you want more amazing breakdowns subscribe!!
Sources
If you wish to further explore the subject here are everything I based this research on
Decisions on previous similar cases
Effects of coating roughness and biofouling on ship resistance and powering
European Journal on Medetomidine 2025
Marine Coatings, Biocides, and Environmental Regulations
Medetomidine: Large consultation response
Disclaimer
The material and information prepared by Simeon Research should not be considered as investment advice of any kind. Each investment decision is made independently and at one’s own risk. Financial instruments can both increase and decrease in value, and there is a risk that you may not recover the invested capital.
Simeon Research owns shares in the company, I will not buying or selling equities in ITECH for at least 30 days since this research is published. I haven’t made any transaction prior to the release of the paper. Last time transaction (buy) was at 23rd of August, I don’t have to disclose that but as always I am fully transparent.
This analysis is independent but financed. This means that Simeon Research has received payment from the Company to prepare the analysis. Simeon Research reserves the right for any factual errors, misprints, or misinterpretations in the analysis.



Thank you very much, very interesting, but Q2 earnings were really bad.
Interesting article highlighting the tension between the potential negative environmental impacts from paints used to tackle hard fouling, but the positive environmental impacts from efficiency gains linked to barnacle free hulls.
The science falls outside my circle of competence to give an informed view, but it was an enjoyable read!